商品名称:QIAseq FastSelectrRNA Yeast Kit (24)
QIAseq FastSelect –rRNA Yeast Kits use a novel method to remove highly abundant RNA that is of low scientific value from your RNA-seq libraries. Researchers using RNA-seq for whole transcriptome analysis can use QIAseq FastSelect –rRNA Yeast Kits to remove cytoplasmic (35S [made up of 25S, 18S and 5.8S], 25S, 18S, 5.8S and 5S) and mitochondrial (21S, 15S, ACI60_gr01 [large subunit ribosomal RNA] and ACI60_gr02 [small subunit ribosomal RNA]) rRNA.
Analyze RNA-seq data with ease using the GeneGlobe-integrated RNA-seq Analysis Portal – an intuitive, web-based data analysis solution created for biologists and included with this QIAseq Kit.
We recommend using QIAseq Stranded RNA Library Kits for robust strand-specific RNA-seq library preparation for both high-quality and highly fragmented RNA.
Design your own custom QIAseq FastSelect pools to remove any RNAs you wish from your RNA-seq library – take a look at our QIAseq FastSelect Custom RNA Removal Kits.
Want to try the QIAseq FastSelect –rRNA Yeast Kit for the first time? Request a trial kit to evaluate.
QIAseq FastSelect –rRNA Yeast Kits demonstrated highly efficient removal of rRNA (figure QIAseq FastSelect –rRNA Yeast Kits provide highly efficient removal of rRNA) and robust performance (figure QIAseq FastSelect ‒rRNA Yeast dramatically improves the number of genes detected).
QIAseq FastSelect –rRNA Yeast Kits also provide consistent read mapping (figures QIAseq FastSelect –rRNA Yeast exon/intron/intergenic mapping, QIAseq FastSelect ‒rRNA Yeast dramatically shifts read allocation from rRNA to genes and QIAseq FastSelect ‒rRNA Yeast dramatically shifts read allocation from rRNA to genes: bar chart) and reproducible gene expression results (figure QIAseq FastSelect ‒rRNA Yeast reproducibility).
 QIAseq FastSelect ‒rRNA Yeast dramatically improves the number of genes detected
QIAseq FastSelect ‒rRNA Yeast dramatically improves the number of genes detected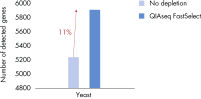 QIAseq FastSelect –rRNA Yeast exon/intron/intergenic mapping
QIAseq FastSelect –rRNA Yeast exon/intron/intergenic mapping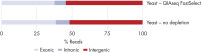 QIAseq FastSelect ‒rRNA Yeast dramatically shifts read allocation from rRNA to genes
QIAseq FastSelect ‒rRNA Yeast dramatically shifts read allocation from rRNA to genes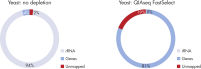 QIAseq FastSelect ‒rRNA Yeast dramatically shifts read allocation from rRNA to genes: bar chart
QIAseq FastSelect ‒rRNA Yeast dramatically shifts read allocation from rRNA to genes: bar chart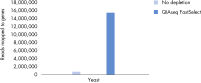 QIAseq FastSelect ‒rRNA Yeast reproducibility
QIAseq FastSelect ‒rRNA Yeast reproducibility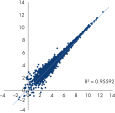
Removing highly expressed, but biologically unimportant yeast RNA transcripts before your RNA-seq library preparation protocol makes NGS more efficient and enables higher sample throughput with higher sensitivity. Furthermore, removal of unwanted RNA species from fragmented RNA samples is particularly challenging and can result in suboptimal performance.
QIAseq FastSelect Yeast Kits are designed for quick, efficient yeast rRNA removal from cytoplasmic and mitochondrial total RNA during NGS RNA library preparation. QIAseq FastSelect seamlessly integrates with your existing RNA stranded library preparation workflow for RNA removal in a single, 14-minute inline step. Prior to RNA heat fragmentation (which is optional and dependent upon the library preparation kit and sample type), QIAseq FastSelect removal reagent is directly combined with total RNA and the library preparation-specific buffers. After fragmentation, the reaction temperature is stepwise cooled to room temperature and the remaining library preparation steps are completed. There is no need to perform any type of enrichment on the total RNA samples. QIAseq FastSelect –rRNA Yeast Kits ensure consistently high performance with RNA amounts ranging from as little as 1 ng to 1 μg. QIAseq FastSelect can be used with RNA from fresh samples, as well as degraded RNA, and delivers reliable rRNA removal and high reproducibility in downstream applications.
Most RNA removal or depletion strategies associated with RNA-seq library construction are sample pre-treatment strategies involving hybrid-capture or enzymatic removal of unwanted RNA. Our unique QIAseq FastSelect procedure is compatible with QIAGEN, Illumina, KAPA and NEB stranded library preparation kits and provides complete rRNA removal in a single, 14-minute inline step (figure QIAseq FastSelect Kit workflow). This is dramatically faster than alternative RNA depletion kits, which require pre-treatment protocols involving more than 25 steps and 2 hours to complete.
Simply add QIAseq FastSelect reagent to the RNA sample, perform fragmentation (if required), stepwise cool the reaction from 75°C to 25°C for 14 minutes and then complete the remaining library preparation steps. QIAseq FastSelect works with or without RNA fragmentation, providing the flexibility to use RNA from degraded RNA samples, or high-quality RNA as part of a standard RNA-seq library construction workflow.