商品名称:Antisense LNA GapmeR in vivo LS (B)
Antisense LNA GapmeRs are highly potent, single-stranded antisense oligonucleotides (ASO) for silencing of lncRNA and mRNA in cell cultures and even in animal models. Antisense LNA GapmeR Positive Controls enable optimization of conditions for lipid-based transfection, electroporation or unassisted delivery. The GapmeRs are enhanced with LNA technology and are designed using sophisticated and empirically developed algorithms and offer excellent performance and high success rates.
Need a quote for your research project or would you like to discuss your project with our specialist team? Just contact us!
 Silencing of mRNA and long non-coding RNA using Antisense LNA GapmeRs.
Silencing of mRNA and long non-coding RNA using Antisense LNA GapmeRs.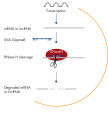 Efficient knockdown with Antisense LNA GapmeRs, regardless of RNA target type and subcellular localization.
Efficient knockdown with Antisense LNA GapmeRs, regardless of RNA target type and subcellular localization. LNA GapmeRs can be used without a transfection agent.
LNA GapmeRs can be used without a transfection agent.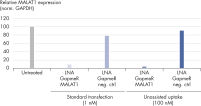 Performance of Antisense LNA GapmeR Positive Controls.
Performance of Antisense LNA GapmeR Positive Controls. Efficient in vivo knockdown with LNA GapmeRs in a broad spectrum of tissues.
Efficient in vivo knockdown with LNA GapmeRs in a broad spectrum of tissues.
Antisense LNA GapmeRs are powerful tools for protein, mRNA and lncRNA loss-of-function studies. These single-stranded, antisense oligonucleotides (ASOs) catalyze RNase H-dependent degradation of complementary RNA targets. The Antisense LNA GapmeRs are 16 nucleotides long and are enriched with LNA in the flanking regions and DNA in an LNA-free central gap, hence the name "GapmeR" (see figure A unique short, single-stranded antisense design). The LNA-containing flanking regions confer nuclease resistance to the antisense oligo, while also increasing target binding affinity, regardless of the GC content. The central DNA "gap" activates RNase H cleavage of the target RNA upon binding. Antisense LNA GapmeRs have fully modified phosphorothioate (PS) backbones, which ensure exceptional resistance to enzymatic degradation.
Antisense LNA GapmeRs are designed using our empirically derived design tool that incorporates more than 20 years of experience with LNA design. For each RNA target, the tool evaluates thousands of possible designs against more than 30 design parameters to identify the Antisense LNA GapmeRs most likely to provide potent and specific target knockdown.
The primary design parameters include the following: